dupilumab conjunctivitis treatment
Treatment options include topical corticosteroids and topical calcineurin inhibitors. Volume 17 Issue 5 May 2019.
All received a prescription of trehalosehyaluroate tear substitute to hydrate conjunctival mucosa.

. However researchers noted that patients who receive. Further patients with more severe baseline AD are more likely to develop conjunctivitis and other ocular complications according to the study. Up to 1 in 5 people taking dupilumab develop conjunctivitis which can cause itching redness and dryness of the eye.
Dupilumab is the first biologic approved for use in treatment of moderate to severe atopic dermatitis AD 1 2 3 4 5. Investigators identified 20 cases of. October 12 2018 Reference ID.
Treatment options include topical corticosteroids and topical calcineurin inhibitors. While the rate of adverse events on dupilumab is generally low mild-to-moderate conjunctivitis associated with redness as well as a burning and foreign body sensation has been reported in up to 28 of patients. Dupilumab may cause conjunctivitis.
Data from phase phase 2 and 3 studies have revealed that dupilumab generally has a low rate of adverse events although an increased incidence of mild-to-moderate conjunctivitis has. Mepolizumab is an IL-5 antagonist antibody also used to treat SEA. The present review highlights the clinical presentation of dupilumab-associated conjunctivitis and addresses pharmacological and non-pharmacological options available for the treatment of this clinically highly relevant condition.
About 4 years after dupilumabs approval were interested in how conjunctivitis has played out in our daily clinical practice lead study investigator Maria C. Current medical management of dupilumab-induced conjunctivitis is topical steroids or cessation of therapy. Conjunctivitis a predisposition to keratoconus and anterior subcapsular cataracts are considered minor criteria of AD.
Trial evidence of more than 1500 people shows that dupilumab is a well-tolerated treatment. Glaucoma cataracts posterior subscapular and and infectious keratoconjunctivitis may complicate therapy with corticosteroids. At baseline 9 75 of the 12 patients had severe AD.
Design Setting and Participants Case series of 12. Dupilumab is approved for the treatment of moderate-to-severe AD moderate-to-severe eosinophilic or oral corticosteroid-dependent asthma and chronic rhinosinusitis with nasal polyps. This is usually mild and usually treated without.
Very few patients stopped taking the medication due to side effects. Dupilumab binds to the alpha-chain of the interleukin IL-4 receptor thus inhibiting signaling of IL-4 and IL-13. While conjunctivitis was a significant adverse event occurring in 3 to 14 of patients with atopic dermatitis AD who received biweekly dupilumab treatments in phase 3 trials vs.
Importance Clinical trials of dupilumab for atopic dermatitis AD have reported an increased incidence of conjunctivitis in patients who received dupilumab compared with those who received placebo. Treatments included antifungal corticosteroid cream itraconazole tablets and tacrolimus 01 ointment. The mean age at conjunctivitis onset was 30 years.
One in 15 patients who start dupilumab treatment may develop conjunctivitis during the first 6 months most of which is manageable with ophthalmic treatments results from large study of US. The conjunctivitis occurred at a mean of 158 weeks of treatment range 8-41 weeks and was considered severe or moderate-to-severe in 4 patients. Long-term safety data beyond one year is still being collected.
In all of the clinical trials analyzed patients receiving dupilumab had a significant increase in conjunctivitis 86 to 221 vs the placebo group 21 to 111. 1 While considered a safe long-term medication for the treatment of these conditions dupilumab has been associated with conjunctivitis of varying severity. Dupilumab was stopped in 3 patients all of whom had severe conjunctivitis.
Treatment was efficacious and safe in all AD phenotypesparticularly in diffuse eczemawith just more than 20 of patients reporting adverse events AEsconjunctivitis 108 flushing 36 followed by injection-site reaction fatigue. A number of treatment protocols for conjunctivitis in dupilumab-treated patients with AD have been proposed in the literature including warm compresses artificial tears eyedrops or ointments with antihistamines anti-inflammatories corticosteroid drops and ointments anti-infective therapies calcineurin inhibitors topical cyclosporine A and. Adverse effects associated with dupilumab include an elevation in peripheral blood eosinophils and severe conjunctivitis the mechanism of which is unknown.
The present review highlights the clinical presentation of dupilumab-associated. Objective To describe the characteristics of patients who develop conjunctivitis secondary to dupilumab treatment for AD. BLA 761055S-042 Dupixent dupilumab injection 4 Version date.
Dupilumab targets interleukin IL-4.

Dupilumab And The Risk Of Conjunctivitis And Serious Infection In Patients With Atopic Dermatitis A Propensity Score Matched Cohort Study Journal Of The American Academy Of Dermatology

Dupilumab An Opportunity To Unravel In Vivo Actions Of Il 4 And Il 13 In Humans Sciencedirect

Schematic Representation Of Pathways Influenced By Dupilumab Treatment Download Scientific Diagram
Conjunctival Cicatrisation In Patient On Dupilumab Patient On Download Scientific Diagram

Transient Increase In Circulating Basophils And Eosinophils In Dupilumab Associated Conjunctivitis In Patients With Atopic Dermatitis Html Acta Dermato Venereologica

Dupilumab Associated Ocular Surface Disease Incidence Management And Long Term Sequelae Medrxiv

New Insights On Ocular Surface Disease In Patients With Atopic Dermatitis Treated With Dupilumab Bortoluzzi 2022 British Journal Of Dermatology Wiley Online Library

Long Term Follow Up And Treatment Outcomes Of Conjunctivitis During Dupilumab Treatment In Patients With Moderate To Severe Atopic Dermatitis The Journal Of Allergy And Clinical Immunology In Practice
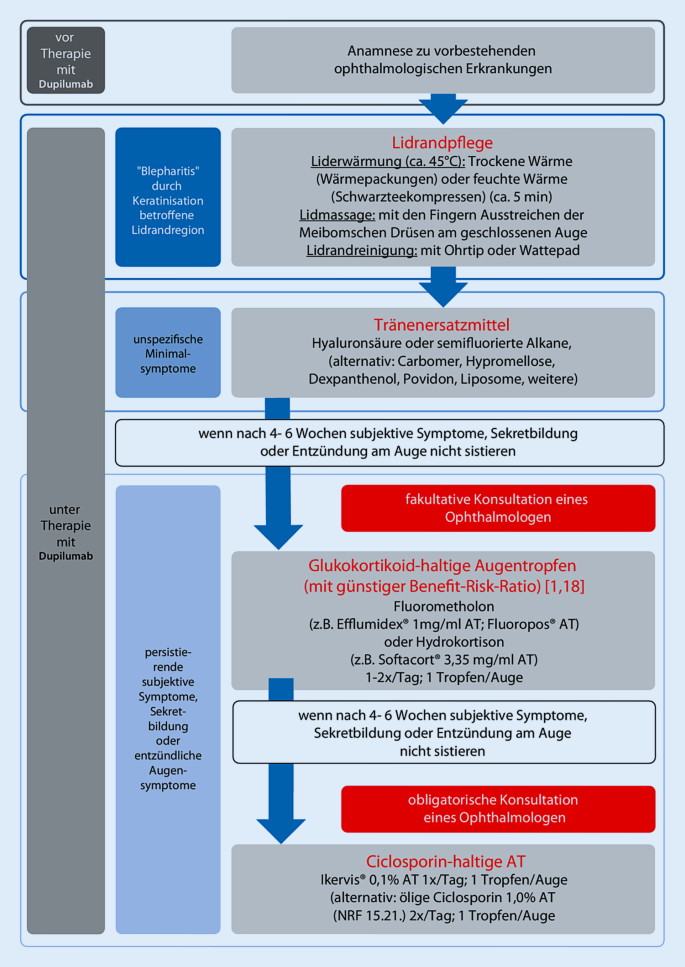
Dupilumab Zur Behandlung Der Therapierefraktaren Atopischen Dermatitis Springerlink

A A Man Aged 46 Years 6 Weeks After Starting Dupilumab Marked Download Scientific Diagram
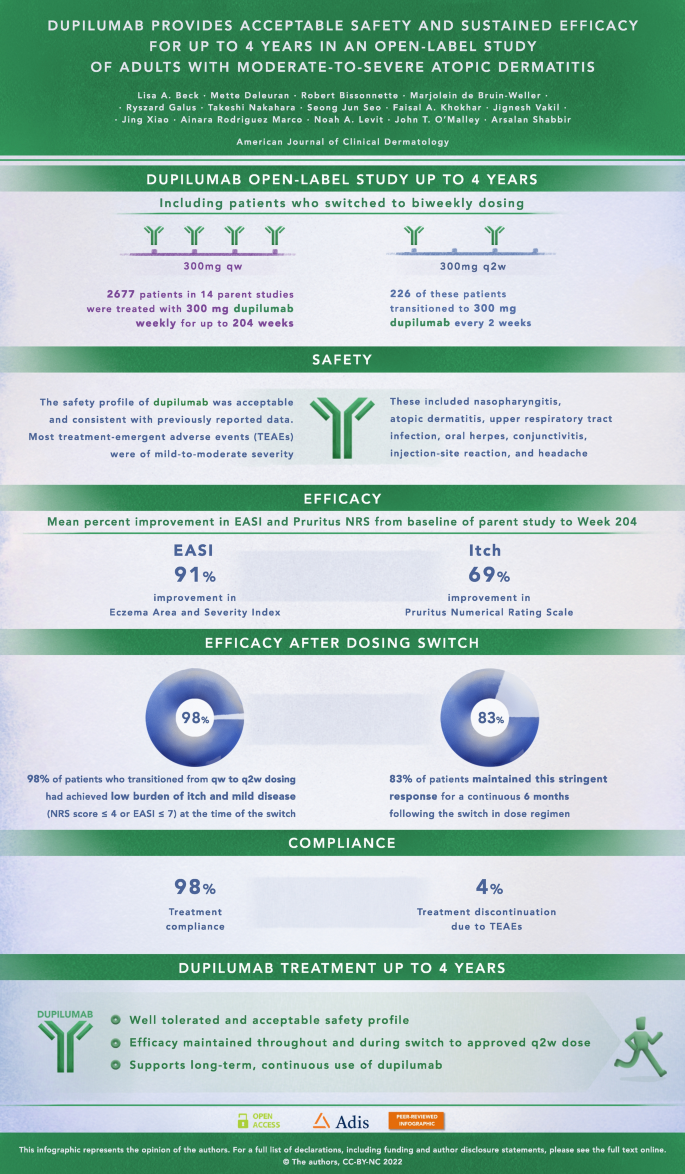
Dupilumab Provides Acceptable Safety And Sustained Efficacy For Up To 4 Years In An Open Label Study Of Adults With Moderate To Severe Atopic Dermatitis Springerlink

Dupilumab Associated Ocular Surface Disease Incidence Management And Long Term Sequelae Medrxiv

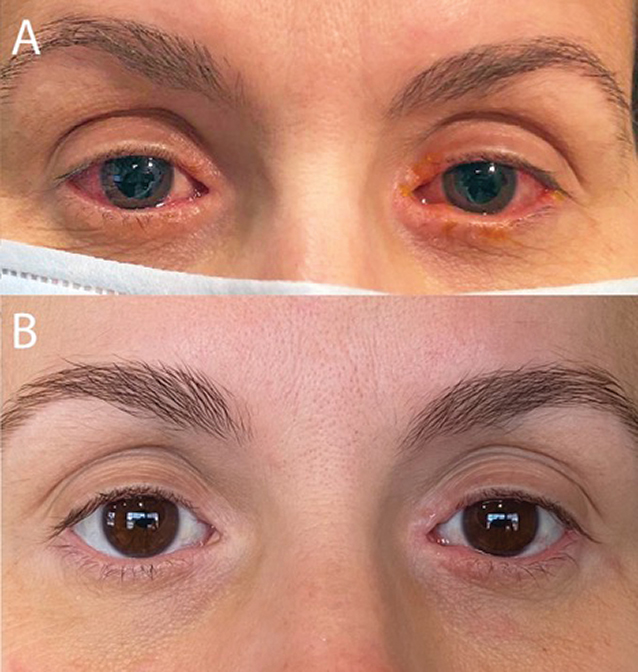
Example Of Case Before And After Treatment With Dupilumab 300mg Every Download Scientific Diagram

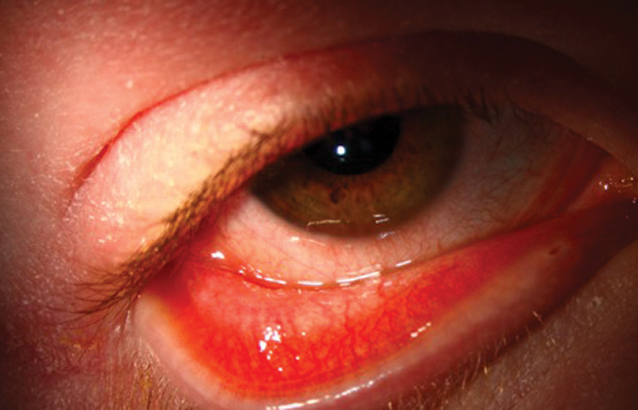
Moderate Conjunctivitis And Blepharitis At Week 22 Of Dupilumab Treatment Download Scientific Diagram

Dupilumab Senkt Den Juckreiz Und Hebt Die Lebensqualitat Rosenfluh Ch

A A Man Aged 46 Years 6 Weeks After Starting Dupilumab Marked Download Scientific Diagram

Comments
Post a Comment